Janma Gene New Corona Epidemic Detection Integrated Solution
With the continuous evolution of the global epidemic, epidemic prevention and control has entered a stage of normalization. In order to properly respond to the current situation and meet the nucleic acid testing requirements of different scenarios, it is necessary to speed up research and development and production organization, and launch a more integrated and suitable for on-site testing. Improve the product and technical system of nucleic acid detection.
Qingdao Janma Gene Technology Company independently developed a new generation of nucleic acid rapid detection platform—ASEA technology, which is accurate, simple and fast. level” significant improvement. It has been successfully applied to many fields such as medical health, food safety, animal disease and so on.
Since the early stage of the epidemic, the Janma Gene Technology Company team has developed a new coronavirus nucleic acid detection kit for the first time. On March 13, 2020, the product obtained the EU CE certification. In June, it entered the white list of the Ministry of Commerce of China, with complete export qualifications. At present, the rapid nucleic acid detection products for COVID-19 have been exported to Indonesia, Brazil, Zambia and other countries, and have been recognized by customers.
Qingdao Janma Gene’s new crown integrated solution products include nucleic acid rapid extraction reagents, new crown nucleic acid detection kits, and detection instruments.
Nucleic Acid Rapid Extraction Reagent
Product introduction:This product integrates the functions of sample preservation, virus inactivation and nucleic acid lysis, which can quickly inactivate the active virus that may exist in the nasal/pharyngeal swab samples. The lysis process does not need to open the lid to avoid secondary pollution caused by opening the lid. This product is easy to operate. After swab sampling, it is directly put into this reagent, and no nucleic acid extraction operation is required. The sample DNA/RNA processed by this product can be directly used in RT-PCR, ASEA and other molecular biology experiments.
Opration Steps: Mix the sample with this reagent → treat at 95°C for 3 min → configure the amplification system as the template after treatment.
Product Advantages:
1. Simple and convenient: simple operation, easy to use, no need to extract, direct sample amplification, and can be matched with amplification reagents from any manufacturer;
2. Fast and efficient: it is used now, and it can be quickly extracted in 3 minutes, with high extraction efficiency and good preservation performance of sample RNA;
3. Safety: It has the functions of sample preservation, virus inactivation, and nucleic acid release, and there is no need to open the lid twice to avoid contamination.
Novel coronavirus nucleic acid detection kit (rapid PCR fluorescence method)
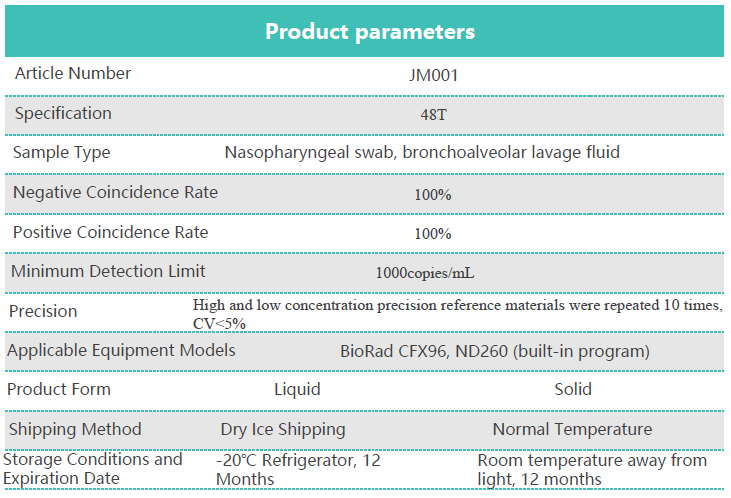
Product introduction: This kit is used for in vitro qualitative detection of new coronavirus infection in the throat swabs and bronchoalveolar lavage fluid samples of suspected cases of pneumonia, suspected cluster cases, and other patients who need to be diagnosed or differentially diagnosed with new coronavirus infection. ORF1ab gene and N gene of 2019 nCoV. Solid reagents and liquid reagents meet the needs of different and storage scenarios.
Product parameters: Please refer to the sheet below
Product Advantages:
1. Simple operation, suitable for different detection scenarios;
2. Rapid detection, nucleic acid amplification in 30 minutes;
3. Support the transportation of solid reagents at room temperature and reduce logistics costs.
Real-time Fluorescence Quantitative PCR Detector (ND360)
Features:
【Accurate】 Temperature control accuracy of ±0.1℃, high-quality temperature control and optical components.
【Fast】 The temperature rise and fall up to ±6.0℃, and the test results are displayed in real time.
【Portable】Small size, light weight, dual-channel dual-module can run independently and double the detection efficiency to meet the needs of inspection and release.
【Easy to use】 Touch color screen, Chinese and English operating system, one-click modular program.
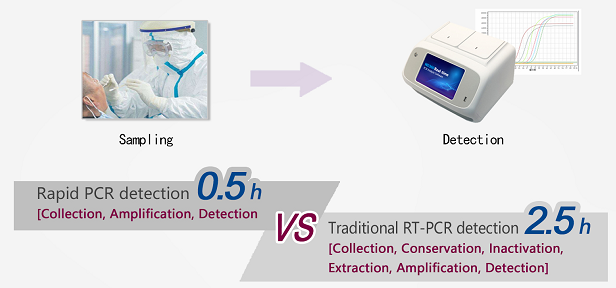
0.5-Hour Integrated testing Recommended Solution
Instrument: ND360 real-time fluorescence quantitative PCR instrument;
Extraction: JM101 New Coronavirus Nucleic Acid Quick Extraction Kit;
Detection: JM001 New Coronavirus Nucleic Acid Detection Kit.
Post time: Mar-03-2022